anda biologicals tb elisa kit|Comparison of A60 and three glycolipid antigens in an ELISA test : import The Elisa test for diagnosis of tuberculosis using highly purified A 60 antigen extracted from mycobacteria was developed by Anda Biologicals, France, during the late 1980s. It is claimed . A key component to understanding what can and cannot be autoclaved is whether or not the material can withstand the pressure of an autoclave’s sterilization cycle.
{plog:ftitle_list}
The study does demonstrate that silica can be used to desiccate an N95, removing moisture that may be generated during the decontamination process using an autoclave or .
The three kits evaluated included the InBios Active TbDetect IgG ELISA (InBios International, Seattle, WA), the IBL M. tuberculosis IgG ELISA (IBL-Hamburg, Hamburg, Germany), and the . Three commercially available serologic assays were evaluated for detection of antibodies in active TB infection namely, InBios Active TB detect IgG ELISA, IBL M. tb IgG .
Objectives: To compare the diagnostic usefulness in tuberculosis of the serodiagnostic enzyme-linked immunosorbent assay (ELISA) kit A60 (Anda Biologicals, Strasbourg, France) and of .Download scientific diagram | ROC curves for the InBios Active Tb Detect IgG ELISA (solid), IBL M . tuberculosis IgG ELISA (dotted), and Anda Biologicals TB ELISA (dashed) for healthy and .The Elisa test for diagnosis of tuberculosis using highly purified A 60 antigen extracted from mycobacteria was developed by Anda Biologicals, France, during the late 1980s. It is claimed . The positivity rates for InBios Active TbDetect ELISA, IBL M. tuberculosis ELISA, and Anda Biologics TB ELISA in latently infected individuals positive by TST and/or QFT-G .
ELISA kit, and 1.2 U/ml for the Anda Biologicals TB ELISA kit). FIG. 2. ROC curves for the InBios Active Tb Detect IgG ELISA (solid), IBL M. tuberculosis IgG ELISA .
Methods: The Pathozyme-Myco IgG (Myco G), Pathozyme TB Complex Plus (TB Complex), IBL M. tuberculosis IgG ELISA (IBL), Anda Biologicals TB IgG (Anda-TB), and T-SPOT.TB (T .“Mycobacteria Patho-tb” rapid diagnostic kit, positive and negative controls (in freeze-dried format with sodium azide), solutions for sample processing; neutralizing and dissolving, and other .
For example, Anderson et al. contributed three studies evaluating three serological tests: (1) InBios Active TbDetect IgG ELISA (InBios International); (2) IBL M. tuberculosis IgG .
Serodiagnosis by ELISA has been widely explored over the years, in the diagnosis of tuberculosis. Two ELISA systems were evaluated for detection of mycobacterial antibodies .
ELISA kits. Group III serum samples were collected from 25 individuals who had received the Mycobacterium bovis bacillus Calmette-Gue´rin (BCG) vaccine. The majority . The Anda .The agreement, sensitivity, and specificity of the Anda Biologics TB ELISA were 74.2%, 83.3%, and 72.0%, respectively. The sensitivity for detecting M. tuberculosis antibodies in human .
“Mycobacteria Patho-tb” rapid diagnostic kit, positive and negative controls (in freeze-dried format with sodium azide), solutions for sample processing; neutralizing and .Three commercially available serologic assays were evaluated for detection of antibodies in active TB infection namely, InBios Active TB detect IgG ELISA, IBL M. tb IgG ELISA and Anda .
In India, at least 13 different TB serological kits are on the market (Table 1), and an estimated 1.5 million serological tests for active TB are performed every year (primarily in the private . The most commonly used antigens are lipoarabinomannan (LAM, MycoDot™ serologic test), antigen 5 (38 kDa, Pathozyme-TB™ ELISA test and ICT diagnostics™), LAM .
The agreement, sensitivity, and specificity of the Anda Biologics TB ELISA were 74.2%, 83.3%, and 72.0%, respectively. The sensitivity for detecting M. tuberculosis antibodies in human .In order to assess the diagnostic usefulness of the A60 (ANDA Biologicals, Strassbourg, France) sero-diagnostic enzyme-linked immunosorbent assay (ELISA) kit for tuberculosis in Africa, .TB Correlative IFN-γ Release Assay (TB-IGRA) TB-IGRA ELISA kit is an enzyme-linked immunosorbent assay kit for the quantitative detection of Interferon Gamma (IFN-y) that .
Download Table | Summary of positive results of InBios Active TbDetect IgG ELISA, IBL M. tuberculosis IgG ELISA, and Anda Biologicals TB ELISA from latently infected individuals and .
with an ELISA KIT (Anda TB Biological, Strasbourg, France). Stored serum samples of our study popula-tion were taken out from the freezer one-hour prior to the test. Anti A60 IgG were .Several antigens from M. tuberculosis have been developed into commercial kits, like MycoDot kit (which uses lipoarabinomannan [LAM]), InBios Active TbDetect IgG enzyme-linked .
The components of the kit will remain stable through the expiration date indicated on the label and package when stored at 2-8°C, do not freeze. To assure maximum performance of this TB IgG .Objectives: To compare the diagnostic usefulness in tuberculosis of the serodiagnostic enzyme-linked immunosorbent assay (ELISA) kit A60 (Anda Biologicals, Strasbourg, France) and of .
ELISA kits are commonly used to measure the concentration of specific proteins, cytokines, hormones, and other biomarkers in biological samples such as serum, plasma, cell culture . The positivity rates for InBios Active TbDetect ELISA, IBL M. tuberculosis ELISA, and Anda Biologics TB ELISA in latently infected individuals positive by TST and/or QFT-G .In order to assess the diagnostic usefulness of the A60 (ANDA Biological, Strassbourg, France) sero-diagnostic enzyme-linked immunosorbent assay (ELISA) kit for tuberculosis in Africa, .
One such serological test is the Anda-TB test developed by Anda Biologicals, which looks for the presence of either IgG, IgA, or IgM antibodies specific for the M.tb A60 antigen. .Measles ELISA Kit. High sensitivity ELISA kit for detection of Measles. Backed by our 100% Guarantee. . AIDS, tuberculosis (TB), malaria, diarrhoeal and respiratory infections account .
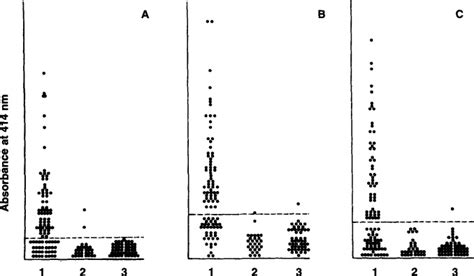
Comparison of A60 and three glycolipid antigens in an ELISA test
Assessment of Three Commercially Available Serologic Assays
I've never seen something that needed to be dissolved before going into the autoclave. You also do not need a stir bar for pouring plates. If pouring plates just give it a swirl when you pull your .I would just take it out before the agar solidified but yes you can autoclave them unless otherwise stated by the manufacturer. Yes and shouldn't have an effect on media, but pretty easy to remove prior with another magnet as you may need it
anda biologicals tb elisa kit|Comparison of A60 and three glycolipid antigens in an ELISA test